Chemical Safety
Chemicals are used across Toronto Metropolitan University for both research and teaching applications. These chemicals have different hazards associated with them (e.g. fire or caustic, asphyxiants, compressed gas vessels under pressure, etc.) that can have the potential to cause harm to students, staff, faculty and the environment. TMU’s Chemical Safety Program is administered by the Environmental Health and Safety (EHS) department. The program was developed to:
- assist and improve user knowledge of chemical hazards;
- ensure the safe handling of chemicals to reduce the health and safety risk;
- meet regulatory compliance; and
- protect students, faculty, staff, visitors and the environment from exposure to hazardous chemicals.
EHS has created multiple tools to help supervisors and users manage their chemical hazards as listed below. For additional information on each tool please continue browsing this website and the resources below.
Key resources
Chemical Safety Manual
The (google doc) Chemical Safety Manual (external link, opens in new window) , available to TMU students, faculty, and staff members, provides health and safety information on the entire lifecycle of each chemical stored on site. This includes delivery, inventory, labelling, use, storage, record-keeping requirements and disposal. The manual also outlines the requirements and procedures established by the university for working with chemicals and compressed gases, as well as roles and responsibilities for supervisors, employees and students who come into contact with them as part of their role.
Laboratory Safety Manual
The (google doc) Laboratory Safety Manual (external link, opens in new window) , also available to students, faculty, and staff members, provides standardized operating procedures for common work practices for lab and chemical handling.
Standard Operating Procedures
(google doc) Laboratory Standard Operating Procedures (SOPs) (external link, opens in new window) are written instructions that detail the steps to be performed during a given experimental procedure and include information about potential hazards and how these hazards will be mitigated. EHS has developed SOPs templates to assist laboratory users with developing operational procedures for common processes and equipment care. Laboratory Supervisors and users can follow these SOPs or customise them to match the specifics of their laboratory. SOPs should be written by laboratory personnel who are most knowledgeable and involved with the experimental process.
Training overview and instructions
Training is a key component of the Chemical Safety Program and it is mandatory for all students, faculty and staff to ensure safe chemical practices are known prior to use. The table below lists the training required depending on your scope of work.
For course instructors, EHS has the ability to enroll your classlist into any of the training modules below. In academic courses that require the use of chemicals, it is strongly recommended that students take the WHMIS Training.
The list below identifies the required training requirements based on the type of work completed:
Scope of work |
Training required |
How to receive the training |
|---|---|---|
Note: WHMIS is mandatory for all university employees including student trainees |
For employees: WHMIS for Employees via D2L Brightspace (30-45 minutes) For students: WHMIS for Students via D2L Brightspace (30-45 minutes) |
|
|
Online Chemical Safety via D2L Brightspace (30 minutes) |
See instructions below |
|
Online Compressed Gas Cylinder Training via D2L Brightspace (45 minutes) |
See instructions below |
|
Transportation of Dangerous Goods (TDG) |
|
|
Vertere (Chemical Inventory System) Training |
|
|
Video tutorial |
To access the training modules, follow these steps:
Step 1: Sign up for a Learner Profile
To sign up for either training, follow these enrollment instructions.
Step 2: Complete the training in D2L
At the end of the training modules, there are quizzes to test participants on what they have learned. Please print or email your certificate and provide it to your supervisor.
WHMIS
The Workplace Hazardous Materials Information System (known as WHMIS) is a Canada-wide standard of communicating information about hazardous products in the workplace. The purpose of WHMIS is to ensure consistent and comprehensive health and safety information about hazardous products are being communicated from the supplier to employees.
Under WHMIS, supervisors are responsible for ensuring:
- employees complete the required training (see table above);
- employees understand where to access SDS and become familiar with them;
- that the most up to date SDS are readily available to those that may be exposed to hazardous products; and
- that workplace labels are correctly applied (see below for further information on ordering these for your area).
Online Safety Data Sheet Management System (Chemwatch)
SDSs provide detailed information about the hazards associated with the chemical, including recommended handling, storage and emergency procedures. Under WHMIS, it is mandatory that all products classified as “hazardous” are accompanied by a Safety Data Sheet (SDS) and employees and students understand where to access them. It is at the discretion of the supervisor whether or not to use paper copies or an electronic format (via Chemwatch) as long as it can be readily available to employees and students who may be exposed to a hazardous product. They must be located close to the workers and accessible during off hours.
TMU staff and students are able to access an online SDS management system called Chemwatch. There are two types of available SDS within the Chemwatch system, Gold SDS and Vendor SDS:
- Gold SDS are product-specific Safety Data Sheets that are written by Chemwatch’s own team of chemists, hygienists, toxicologists, and regulatory experts. These documents are written with the primary goal of providing comprehensive information for workplace safety and hazard communication. They are primarily pure chemicals or common mixtures, and are not related to or published by any particular vendor who may sell the product. Many research laboratories use these Gold SDSs almost exclusively, since they contain such a wealth of information about the chemicals.
- Vendor SDSs are the Safety Data Sheets that are written and distributed by the manufacturer or supplier of a particular product. These are stored as PDF documents in Chemwatch and will be exact copies of the SDSs you would receive from your vendor when you order a chemical.
SDSs can be found online using any computer on campus by accessing the Chemwatch SDS Database (external link) .
Note: The Chemwatch SDS Database link should be bookmarked on all lab and office computers used by lab participants. It does not require a username or password to access, but you must be on campus (i.e. TMU IP address) for the link to work. If you need access to Chemwatch off site, please contact EHS.
Instructions for using Chemwatch and an explanation of the features offered are outlined in this video tutorial (external link) . Additional help on the Chemwatch system can be accessed via Chemwatch’s comprehensive eLearning video (external link) .
Standard operating procedures
Receiving hazardous materials
Shipping and Receiving at Toronto Metropolitan University maintains a central receiving dock at 105 Bond Street, where all goods must be received. Shipping and Receiving receives, sorts and delivers all goods — including couriered and bulk deliveries and compressed gases — on a regularly scheduled basis to all departments and buildings.
If you need to transport chemicals between labs in non-connecting buildings or off-campus, please visit the TDG page.
Transporting chemicals off-campus must be done in compliance with specific Federal Transportation of Dangerous Goods (TDG) regulations. If you complete this function then you are required to have TDG training.
Toronto Metropolitan University Lab at MaRS and iBEST
Hazardous materials destined for the Toronto Metropolitan University Lab at MaRS or iBEST are sent directly to the MaRS Discovery District, West Tower at 661 University Ave, 11th floor and iBEST at Li Ka-Shing Knowledge Institute, 209 Victoria St, 7th floor respectively.
Managing inventory
The university has recently completed the full implementation of a hazardous materials inventory system called HECHMET (Higher Education Cooperative for Hazardous Materials and Equipment Tracking), which is a cooperative of institutions with similar goals for tracking hazardous materials.
Tracking and tagging with Vertére
The HECHMET project is underpinned by the Vertére Inventory Manager (VIM) software, which is a web-based online chemical inventory system used to track chemicals and other materials within the university environment.
All new hazardous materials being delivered to TMU receive a unique barcode, which is scanned and categorized into Vertére. This process happens upon arrival at Shipping and Receiving and prior to delivery of the materials to your location.
Access the Vertére Inventory Manager (VIM) software (external link) .
This web-based inventory system is easily accessible by users and allows them to review a list of hazardous chemical products and the specific location where they are being used or stored on campus.
Chemicals are tagged with a unique barcode label that cross-references to:
- storage location;
- Principal Investigator (PI) responsible for the item;
- chemical information; and
- SDS-related information, as required.
While the chemical inventory offers various advantages to the entire TMU community, users will also find it to be a valuable tool for tasks such as:
- searching by chemical name, location, room or researcher(s);
- knowing what you have on-hand to prevent waste; and
- exporting or printing search results for future reference.
All Principal Investigators and labs have a unique username and password. Please contact chemical.inventory@torontomu.ca to have an account created.
Tracking and tagging compressed gas cylinders
All delivered compressed gas cylinders must be tracked by receiving a unique barcode, which is scanned and categorized into Vertére. This process happens upon arrival and delivery of full cylinders to one of TMU’s compressed gas storage locations. Laboratories that use compressed gases should follow the steps outlined in (google doc) TMU-LAB-007 - Gas Cylinder Receiving, Transferring and Handling procedure. (external link) This procedure outlines the specific details regarding maintaining inventory, how to return empty cylinders and how to access cylinder storage locations after successful completion of training.
A workplace label is a label that an employer produces, for use in the employer's workplace only. For the chemicals at TMU that require a workplace label, EHS has developed two standard workplace label templates.
Both template 1 and 2 allow the user to supply the following information:
- chemical or generic name
- Chemical Abstracts Service (CAS) number
- if it is a mixture, percentage of each chemical within
- WHMIS symbols and indication if these pose a low (warning) or higher (danger) risk
- required PPE for handling
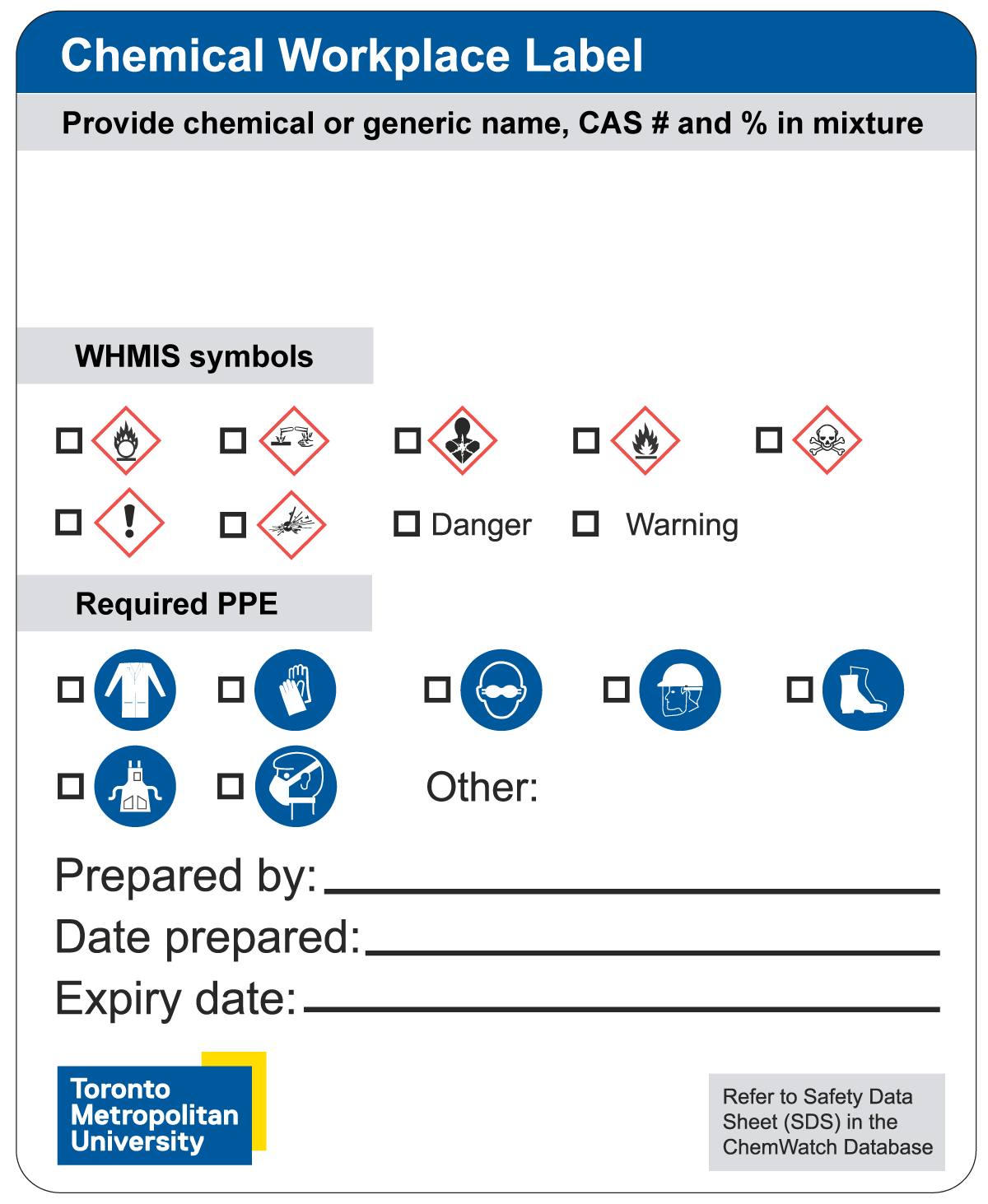
Template 1
This template also includes the following additional fields
- name of who prepared the chemical
- date it was prepared
- expiry date
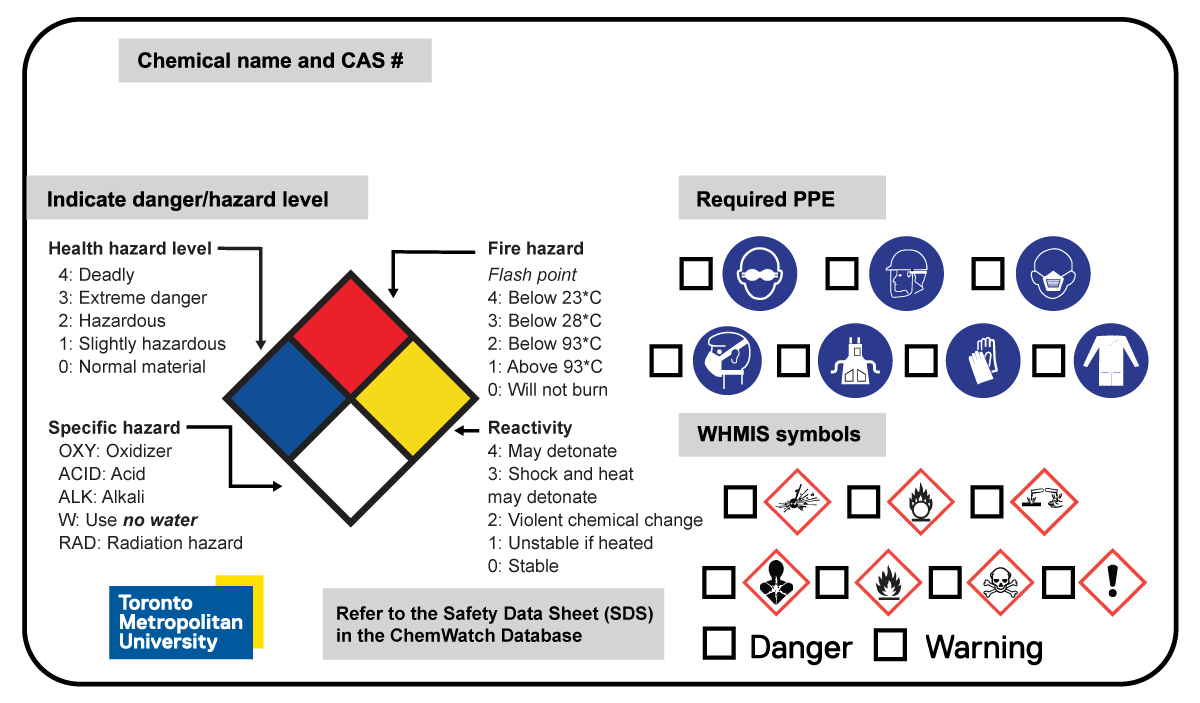
Template #2
This template includes the National Fire Prevention Association (NFPA) rating system. This American system uses a colour-coded diamond with four quadrants in which numbers are used in the upper three quadrants to signal the degree of health hazard (blue), flammability hazard (red) and reactivity hazard (yellow).
Users may choose to use this version of the template when they would like to have a system to compare similar products and their associated levels of danger.
To order chemical workplace labels:
- Visit the online ordering system for TMU's chemical safety labels (external link) .
- Enter the password: “Ryerson” to access the system.
- Click on the desired template and sizes available.
- Select the quantity of labels, these are already chemical and water resistant.
- Select quantity of laminate covers (optional).
- Note: For heavier chemical users, you may want to purchase the laminate covers in addition to the labels to prevent the ink from the markers to fade or be removed from harsh solvents used in the lab.
- Add to cart.
- Apply coupon code: “Ryerson” to all orders over $115 to receive free shipping.
- Checkout.
Labels will be delivered within approximately 5 to 10 business days. If you have any questions, please contact Wholesale Safety Labels (external link) directly.
How to complete chemical workplace labels
- Name the product contents: Provide the chemical or generic name(s) and CAS number(s). If the solution includes more than one chemical, include what percentage of each the mixture contains.
- Check WHMIS symbol(s) that apply
- oxidizing hazard
- may cause (or suspected of causing) serious health effects
- fire hazard
- may cause death or toxicity with short exposure to small amounts
- may cause less serious health effects or damage the ozone layer
- explosion or reactivity hazards
- Indicate risk level: For high risk hazards, check “Danger”. For less severe hazards, check “Warning”. Find more information in section 2 of the Safety Data Sheet (SDS) in the ChemWatch database.
- Indicate what Personal Protective Equipment (PPE) is required while handling the chemical(s)
- lab coat
- safety gloves
- safety goggles or glasses
- face shield
- close-toed shoes
- apron
- mask
- Provide additional information
- Name of the person that prepared and transferred the chemical(s) into the container.
- Date the chemical(s) were prepared and transferred into the container.
- Expiration date of the chemical(s), if applicable.
- Refer to SDS in ChemWatch: Find more information about any chemical by searching their SDSs in the ChemWatch database, including possible health effects and proper disposal methods.
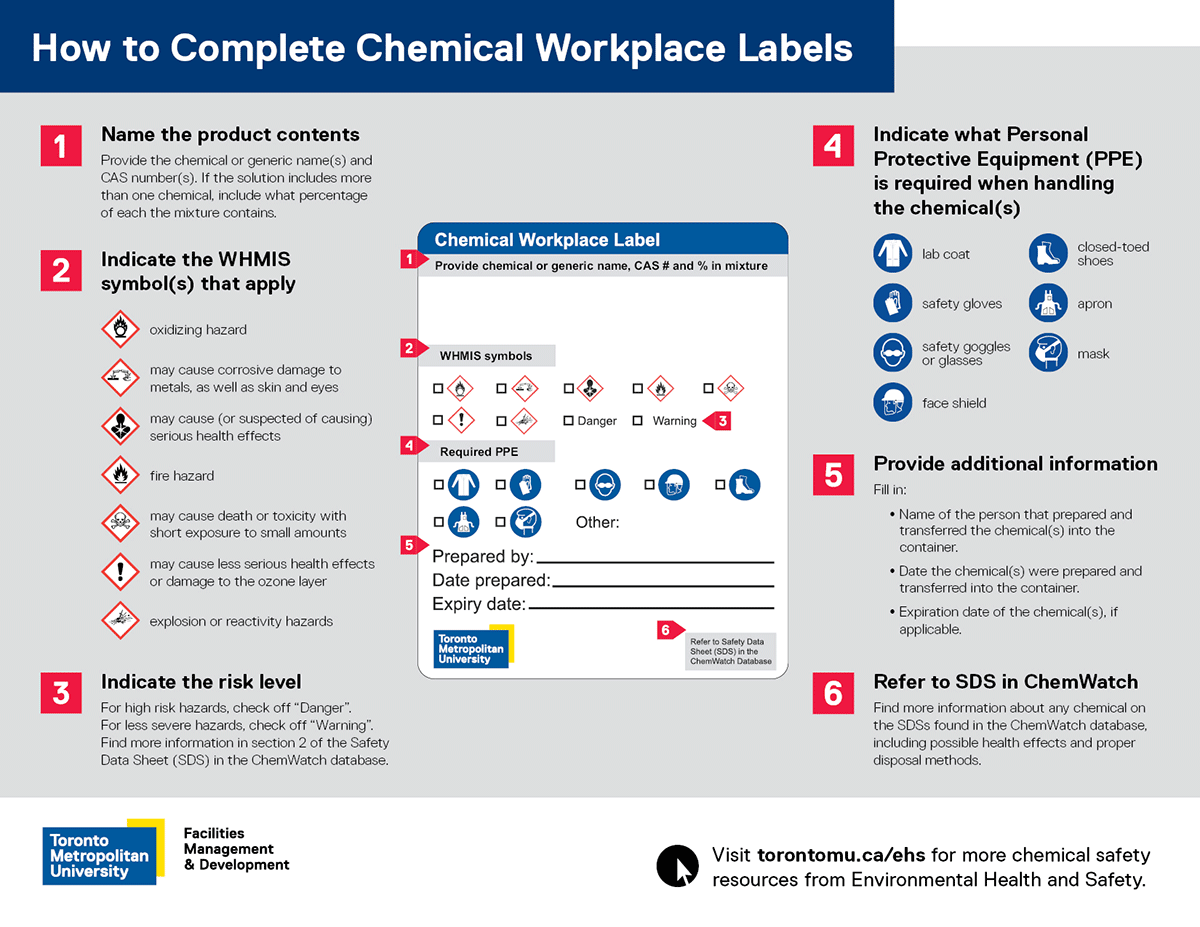
Download and print the (PDF file) How to Complete Chemical Workplace Labels poster which outlines the above instructions for chemical users.
It is critical to dispose of chemical waste properly so as not to cause harm to yourself and the environment. Please follow these steps:
- Collect chemical waste in appropriate leak-proof waste containers. EHS does not supply containers but they can be purchased at various companies, such as Uline, Acklands-Grainger, VWR and Fisher Scientific. Remember not to put incompatible chemicals in the same container, opting instead to use a second container. Keep the container closed at all times to minimize exposure.
- Label the container using the (google doc) Hazardous Waste Disposal Label template (external link) , available to TMU students, faculty and staff for print.
Environmental Health and Safety at TMU is committed to accessibility for persons with disabilities. If you require this document in an alternative format, please get in touch with your department’s EHS contact. - Print the label, fill in the information by hand and affix it to your container.
- Request pick-up. Once your container is filled and labelled, it is ready to be picked up by the hazardous waste company. Complete and submit an online Chemical Waste Disposal Request form for pick-up. When filling out this form, please note:
- After completing your contact information and the location where the waste resides, you will be prompted for information about the chemical. The tag number requested refers to the barcode number that the item has been tagged with for inventory. If you do not have a tag number, leave this field empty. If there is more than one tag number, you can add multiple tag numbers by clicking on “Add” button on the right side.
- Under the Chemical Composition field, please enter the composition of the chemical. This will help in determining the properties of the substance.
- Next you will be required to choose the physical form of the chemical from gas/aerosol can, solid and liquid. You will need to select either the Total Volume or Weight option, depending on the physical form. If you select Volume, you will need to enter the volume in either litres/gallons. If you select Weight, you will need to enter the weight in either kilograms/pounds.
- The last field will ask you whether any sharps are included in the waste. Please note there should be no biological sharps in the chemical waste.
- If there is more than one chemical waste item ready to be picked up, click on the “Add” button to enter the information for the next chemical.
- Submit the form when you are done.
Source: Hazardous Laboratory Chemicals Disposal Guide (3rd Edition) by Margaret-Ann Armour, 2003.
All chemicals and compressed gases arriving on campus are now barcoded and inventoried upon arrival at Shipping and Receiving and prior to delivery to the user. In order to keep the inventory as accurate as possible, any barcoded container from which contents have been emptied must be removed from the inventory. Please follow the simple process outlined below for disposal of empty containers or returning empty compressed gas cylinders.

If your container has a TMU chemical inventory barcode
Chemical container |
Compressed gas cylinder |
|---|---|
Take a picture of the barcode you would like to remove. Send the picture to Dispose of the container as required (e.g. hazardous, glass, recycling, etc.). |
Send a pick up request for your empty cylinder to the supplier and copy Return your empty cylinder to the compressed gas storage location. |
If your container does not have TMU chemical inventory barcode
Chemical container |
Compressed gas cylinder |
|---|---|
Dispose of the container as required (e.g. hazardous, glass, recycling, etc.). |
Send a pick up request for your empty cylinder to the supplier and copy chemical.inventory@torontomu.ca. |
All broken glassware that is not contaminated by chemical or biological agents (e.g. microscope slides, slide covers and Pasteur pipettes) are to be deposited into a broken glass waste container. Wear gloves and use tongs or a dustpan to pick up pieces for disposal, and never pick up a broken chemical glass container with your bare hands. When a broken glass waste container is full, close and seal the box, mark it as garbage and submit a service request to the Facilities Help Desk for a pick-up.
Legislation
The Chemical Safety Program is in compliance by law with the Occupational Health and Safety Act (OHSA) (external link) and the Workplace Hazardous Materials Information System (WHMIS).